By Alan Curtis
The human immune system has two main branches: the innate and the adaptive immune systems. The innate immune system includes simple external defenses such as skin and tears and more complex internal defenses like patrol cells, called neutrophils, that look for anything that might be out of place. The adaptive branch is considered far more complex because it has special cells that “learn” and adapt to attacks on the body by viruses, bacteria, parasites, toxins, and more!
The B cell is a major adaptive immune cell. B cells are formed in the bone marrow throughout our lives and can respond to specific materials produced by pathogens and almost anything else – proteins, fats, sugars, nucleic acids, etc.! This ability to recognize and respond to seemingly endless types of material is due to the B cell’s ability to produce special proteins called antibodies.
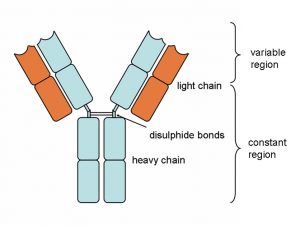
Antibodies are special Y-shaped proteins (Figure 1) that are produced by B cells at all times. In fact, following an infection or a vaccination, special types of B cells, called plasma cells, produce antibodies that fight the pathogen and protect the body from infection. Antibodies made against certain viruses like Rubella can be found in the blood of women during prenatal care visits even though the previous exposure to the virus (or vaccine) was decades earlier! If, for some reason, the woman does not have these antibodies, a booster vaccine can be administered to reboot old B cell clones to make more protective antibodies.
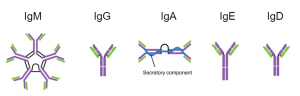
Ok, ok…antibodies are important, but how do they work? As shown in the figure above, these Y-shaped proteins have four overlapping domains. There are two shorter chains (shown in orange) called light chains and two longer chains (blue) called heavy chains. The two chains combine together to form the complete antibody. The top part of the structure interacts with the antigen while the bottom part dictates the function. In humans, there are five different types of antibodies (Figure 2) that all have different roles in the immune response. Antibodies are named using letters of the Greek alphabet (Figure 2). Antibodies that utilize the mu heavy chain, for example, are called immunoglobulin mu (or IgM for short).But how are there so many?
During B cell development, there are highly orchestrated processes that change the DNA of the B cell. The result is an impressive diversity of antibody specificities (viruses, bacteria) and function. Without the gene rearrangement that occurs during B cell development, antibodies would be largely useless and people would become frequently sick and die.
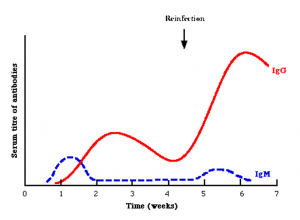
Each of the 5 types of antibodies have distinct functions, and the immune system can use some or all types at once in order to fight off disease. IgM is the first line defender. It appears early in the immune response (Figure 3) and usually has weak specificity for its target (bacterial cell wall, for example). It can exist as a monomer (single antibody, like IgG) or pentamer (5 individual antibodies joined together, like IgM). The monomer form is usually found on the B cell surface only while the pentamer form is found in the blood and tissues. It has broad activity and allows the immune system to get a handle on things. Once the immune response matures, the antibody response can change to IgG, IgA, IgE, IgD, or some combination of all 5.
IgG is the heavy hitter and is responsible for protecting the body against viruses and bacteria. It is the most common type of antibody found in human blood. It can directly bind to viruses and prevent infection (as with measles). This antibody class can eliminate toxins. IgG can also coat bacteria or other invaders, a process called opsonization, that allows other immune cells like natural killer cells and macrophages to eat the bacteria. There are four subtypes of IgG in humans.
IgA is present in mucus, tears, and saliva. It is also found in the intestines, where it it plays the role of gatekeeper by using its secretory component (pictured in Figure 2) to pass between the body’s tissues and the inside of our intestines. By doing this, it can carry bacteria that have managed to cross into our tissues safely back to the intestines without causing damage and inflammation.
IgE interacts with special immune cells called eosinophils. Eosinophils, with help from IgE, are antiparasitic, but they can also cause severe allergy and life-threatening anaphylaxis! IgE is usually found in incredibly low levels and needs to be tested by specialized laboratories. When in high numbers, it can lead to inflammatory diseases like eczema.
IgD, the last major type of antibody, is an enigma, meaning we aren’t really sure exactly what it does. We used to think it was only found on the surfaces. of B cells. In recent years, however, IgD has been detected in the blood. IgD does play a role in B cell development and can also interact with another type of immune cell called basophils, which are involved in histamine release and blood clotting.
So when you hear the term “antibody,” remember that they are a broad class of highly complex immune proteins tasked with keeping us free from disease!
Edited by Keean Braceros and Meryem Ok
