By Riya Gohil

You probably think mosquitoes are a nuisance and wish they didn’t exist, but is wiping out whole mosquito populations really a good idea? Earlier this summer, Oxitec, a biotechnology company, received approval from the EPA to release genetically modified mosquitoes in Florida and Texas. Oxitec aims to use these modified mosquitos to reduce numbers of mosquitoes that cause local outbreaks of diseases like the Zika virus and dengue fever. Oxitec generated a population of male Aedes aegypti mosquitoes carrying a gene that causes their offspring to die before reaching adulthood. These mosquitoes were generated using a technique called gene drive, which modifies genes so they don’t follow traditional hereditary patterns. In this case, gene drive is being used to drastically increase the chances of this ‘early death’ gene being inherited by the offspring mosquitoes.
In normal reproduction, offspring have a 50% chance of inheriting a specific version of a gene, or allele, from either of their parents. Alleles are different variants, or versions, of a single gene. For example, let’s say we have a purple dog and a yellow dog, and that coat color is determined by a single gene. In a normal scenario, their puppy would have a 50% chance of being yellow by inheriting the yellow allele, and a 50% chance of being purple by inheriting the purple allele. In a gene drive, we could control the puppy’s coat color completely and there would be a 100% chance of it being the color we wanted. A gene drive ensures that a particular allele of a gene will always be transmitted to offspring. The lethal tTav gene is Oxitec’s tool to create the gene drive in mosquitoes. Male mosquitoes with tTav pass this gene on to all their offspring. The tTAV gene encodes for the tTAV protein, which increases production of itself to increase the chances of tTAV binding to essential genes. tTAV binds and prevents essential genes from controlling activities necessary for development and growth. Consequently, mosquitoes born with this lethal gene end up dying before they can reach adulthood.
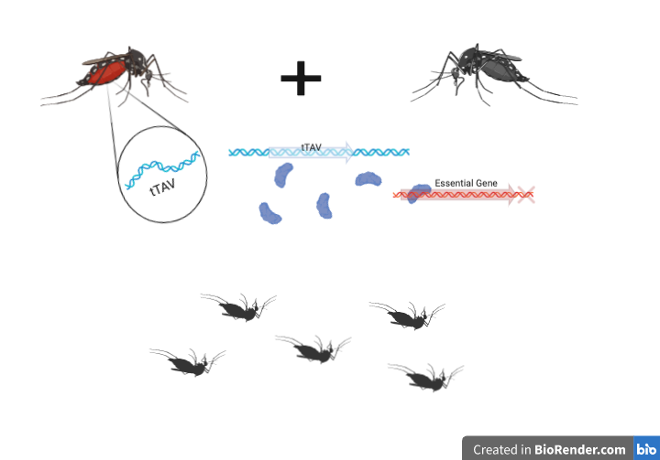
To generate a line of male mosquitoes with the tTAV protein not attacking its own genetic machinery, Oxitec developed an antidote, tetracycline, to feed to these male mosquitoes that inhibit tTAV from shutting off essential genes, and thus preventing the male mosquitoes from dying from their own tTAV gene. Once released in the wild, these male mosquitoes can mate with disease-carrying female mosquitoes causing most of their offspring to die before reaching adulthood and passing on diseases to humans.
This research has the potential to reduce the prevalence of mosquito populations and can lead to a decrease in the cases and spread of mosquito-borne diseases. Reducing disease-carrying mosquito populations can save up to several million lives every year.

While less disease-carrying mosquitoes sounds great, there could be unintended and unforeseeable consequences. For instance, we don’t know how reducing the mosquito population will affect local biodiversity and if the genetic modification could cause harm to humans or animals. A reduction in mosquito population could harm species that have diets that heavily rely on mosquitoes such as amphibians, birds, reptiles, fish, and insects. Scientists so far have introduced these mosquitoes in a controlled environment. They saw no significant developmental effect on animals feeding on these mosquitoes and no byproduct toxin dangerous to humans. Despite these positive results, real life application does not always follow the same results as a test environment. Previous lab tests with Oxitec’s mosquitoes show that up to 3% of offspring survive, but it isn’t clear whether those offspring can produce their own progeny. Another major concern is that while Oxitec does have an antidote to the tTAV protein, these mosquitos can develop mutations to become resistant to the antidote, thus the negative consequences can’t be stopped.
While the works of this research seem promising, there are too many uncontrolled consequences that can arise that we don’t have the proper means of fixing yet.
