By Henry Dieckhaus
Approximately 10% of people identify as left-handed if asked to write a sentence or throw a ball with their preferred hand. However, exactly 100% of people are left-handed where it counts: in the molecules that make up their cells.
How can a molecule be left-handed? To answer this question, we first need to talk about chirality. Hold your hands up in front of you. Are they the same shape? Almost, but not quite. To see what I mean, try to superimpose (line up) your hands so that they look exactly the same. If you place them on top of each other, the thumbs poke out in opposite directions. If you flip one hand and line up each finger, the front of one hand faces the back side of the other. This property is called chirality, and it is the same reason why you can’t shake someone’s right hand with your left hand (at least, not very easily). Two objects (i.e., hands) are considered chiral if they are mirror images that cannot be superimposed on one another, and achiral if they can.
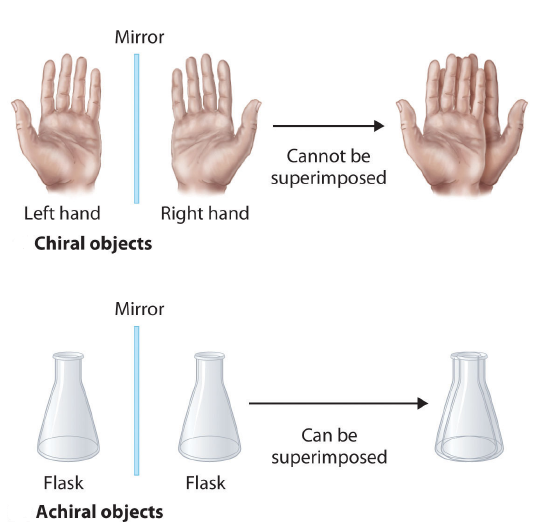
Everyday examples of chiral and achiral objects (Credit: LibreTexts Chemistry, used under a Creative Commons license)
What does this have to do with cells? Well, all kinds of objects can be chiral, including the molecules that make up our cells. Proteins, for example, are chiral molecules that serve many important functions in our bodies. Some work as enzymes, enabling chemical reactions so that we may breathe and digest food, while others work as receptors, detecting chemical signals such as tastes and smells. To make different kinds of proteins, small building blocks called amino acids are linked together like beads on a string. Almost all natural proteins are built using the same set of 20 amino acids, 19 of which are chiral. We can see this by looking at opposite “handed” versions of the same amino acid. Like our hands, they are 3-dimensional, and they cannot be superimposed, so they must be chiral.
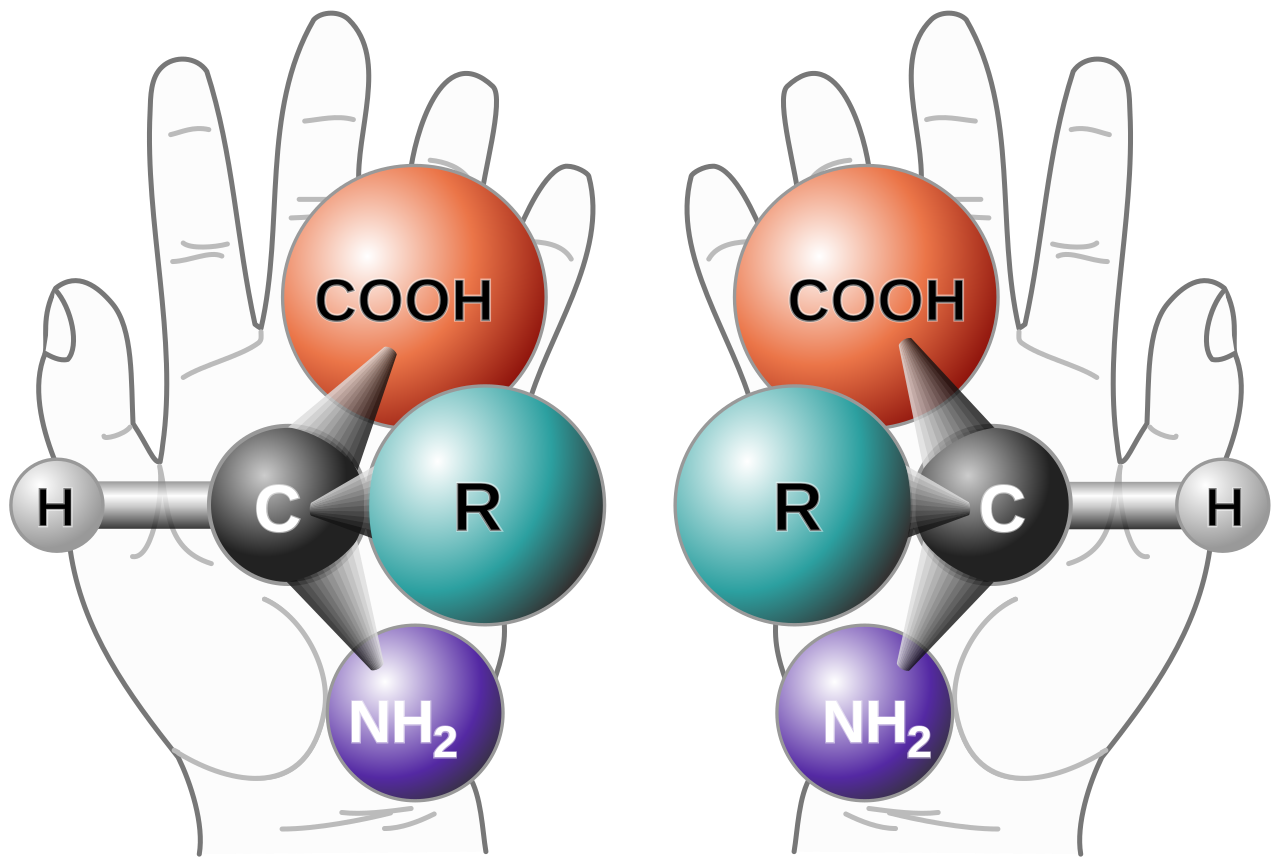
Demonstration of amino acid chirality with a hypothetical generic amino acid (Credit: Courtesy of NASA, in the public domain)
If amino acids are chiral, then proteins must be as well, since proteins are made of amino acids. This means that all proteins must be either left- or right-handed (in chemical terms, either “L” or “D”, respectively). So which is it? At least on this planet, L-amino acids (“left-handed”) are nearly universal across all living organisms, while D-amino acids (“right-handed”) are rarely used in nature.
This evolutionary decision has had a big effect on life as we know it. The chemistry of life is complicated, and many reactions have to happen all at once without interfering with one another. To achieve this, different proteins have evolved to only interact with specific molecules that match their shape just right, like a lock into a key.
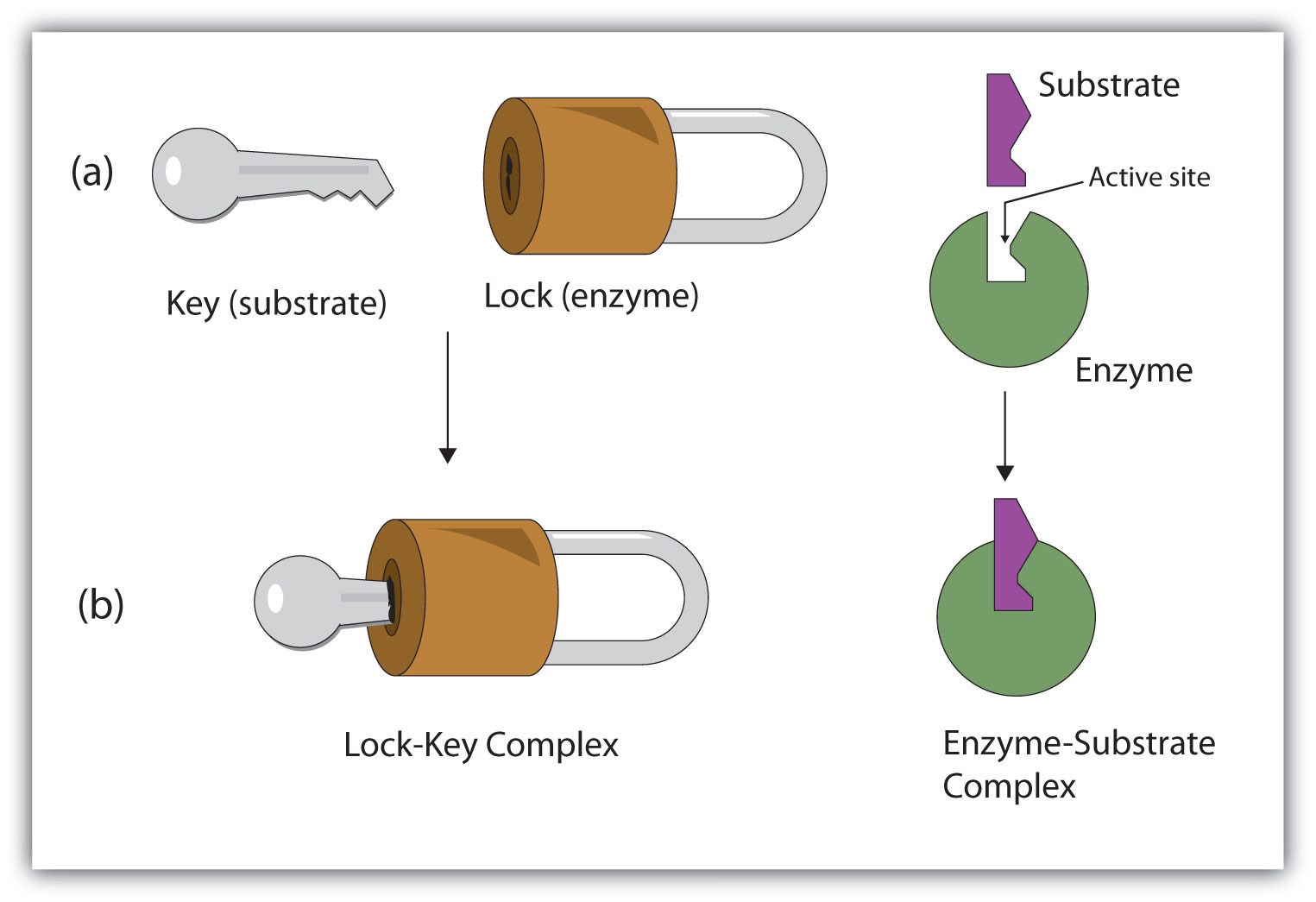
The lock-and-key model of protein binding. In this example, the “lock” is an enzyme (protein), while the “key” is another molecule (a.k.a. the substrate). (Credit: Saylor Academy, used under a Creative Commons license)
Just like you can’t easily put your left shoe on your right foot, many proteins will not bind a molecule that is the wrong “handedness”. For example, the “right-handed” version of the molecule carvone smells like mint, while the “left-handed” version smells like rye bread, since they interact with different olfactory receptors in your nose.
Many questions about the chirality of life remain: Why are proteins left-handed, rather than right-handed? Is “handedness” (chirality) required for life? Scientists are hard at work trying to uncover the answers to these questions. We may never know exactly how life arose from a soup of disordered atoms, but by asking these kinds of questions, we stand to learn a great deal about ourselves and about our world.
