By Macy Osborne-Frazier
Oh no, you spent an hour this morning straightening your hair and after 10 minutes in the humid, summer air, your hair is already starting to curl again. It can seem impossible to keep your hair styled depending on the weather, but there’s actually a simple scientific explanation behind hairstyling. Interestingly, hair texture and styling is all thanks to the proteins that make up the hair structure.
Proteins are encoded for by DNA, which means we all have unique hair structures based on our unique DNA. Hair grows from tiny openings in our skin called hair follicles. The shape of the hair follicle is determined by the proteins that the DNA codes for. If a person’s DNA codes for proteins that make a perfectly round follicle, their hair can grow straight out of the follicle resulting in straight hair (Fig 1). The same is true for other hair textures: follicle shape determines hair structure.
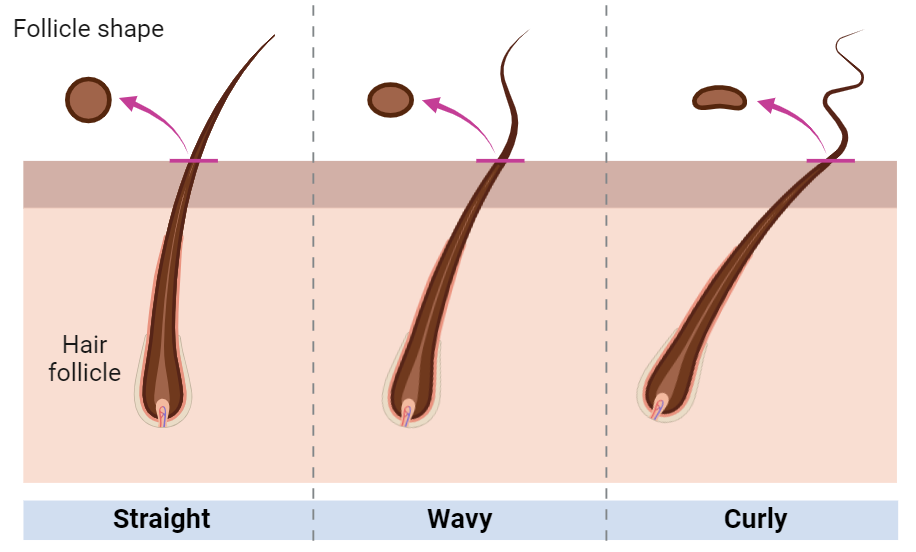
Figure 1. Illustration of hair follicle shapes (Created by author with BioRender.com)
If follicle shape determines how the hair grows, then how do straighteners and curling irons work? Just like before, the answer is proteins. The proteins in our hair help it maintain its texture once it has grown out of our hair follicles. The proteins in our hair bind each other by hydrogen bonds. The hydrogen bonds between the proteins in our hair maintain the shape of hair even once it has grown out far past the follicle. However, hydrogen bonds are easily broken by heat. This means that when we use hot tools like straighteners or curling irons, we break the hydrogen bonds that form our hair texture. If the hydrogen bonds between the proteins in your hair make your hair curly, using a hot straightener breaks those bonds and forces the hair to be straight instead (Fig 2).

Figure 2. Hydrogen bonds in curly hair are broken by heat to make the hair straight (Image source)
Hydrogen bonds are not just broken by heat; they are also broken by contact with water. This is why wavy hair often becomes straight when it gets wet. However, once the hair dries, the original hydrogen bonds reform. The reforming of the original hydrogen bonds after hair becomes wet explains why wet, humid air – or even a rainy day – can cause straightened hair to re-curl or curled hair to straighten back out. This is also why our hair returns to its natural texture after we wash it. Although hydrogen bonds are easily broken, they eventually reform, leaving us with our natural hair texture once again.
To more permanently alter hair structure, techniques using permanent waves (perms) or chemical relaxers are often used to make hair curly or straight for long periods of time. Perms and relaxers also alter the bonds between proteins in hair. However, these types of hair treatments affect a different type of protein bond called a disulfide bond. These bonds are stronger than hydrogen bonds and are not broken by things like heat or water. Instead, the chemicals used in perms and relaxers first attack the disulfide bonds to alter hair structure and then cause new disulfide bonds to form in the desired structure. Since the new disulfide bonds formed from these treatments are strong bonds, the hair structure remains altered for many months.
Hair styling and treatments are a perfect example of how better understanding the underlying biochemistry of our bodies has changed society and trends. Being aware of the types of protein bonds and how those can be altered has allowed us to change and experiment with our hair in new ways.
