by Marco Gontijo
Fun Rating: 4/5
Difficulty Rating: 1/5
What is the general purpose? Antimicrobial resistance (AMR) occurs when organisms, such as bacteria, viruses, fungi, and parasites, resist the effects of antimicrobial drugs, making infections more challenging to treat and increasing the risk of severe illness, complications, and mortality.
The non-profit organization Clinical and Laboratory Standards Institute (CLSI) in the United States and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) in Europe are the leading organizations that standardize the techniques used to assess if microbes are resistant or not to the current drugs available to treat infections.
Antimicrobial susceptibility testing (AST) techniques determine how effective specific drugs are against microbes, guiding treatment decisions and detecting resistance. AST helps clinicians choose the most appropriate therapy and track the spread of antibiotic-resistant bacteria. Additionally, AST is essential in research and drug development, aiding in evaluating new antimicrobial drugs and resistance mechanisms (CLSI & EUCAST). In the case of bacteria, those drugs are called antibiotics.
Why do we use it? A previous publication on our site has explored one type of test known as the Kirby-Bauer Test, the most traditional and widely used method for determining bacterial susceptibility to antibiotics. This post will explain a different and more quantitative technique, the broth dilution assay, with advantages and disadvantages compared to the traditional test (Table 1).
Table 1. Comparison of Antimicrobial Susceptibility Testing Methods at a Glance.
| Feature | Kirby-Bauer Test | Broth Dilution Assay |
| Result Type | Qualitative | Quantitative |
| Growth Medium | Solid | Liquid |
| Antibiotic Application | Drug impregnated in diffusion disks | Drug diluted in medium |
| Interpretation | Measure inhibition zone | Determine action concentrations |
| Advantages | Simple and cost-effective | Precise |
| Disadvantages | Less precise | Requires specialized equipment and labor-intensive |
How does it work? This post will explain the technique with bacteria, and a more detailed breakdown of the protocol and other applications can be found here:
Bacterial Culture Preparation:
- Bacterial Isolation: A pure bacterial culture is obtained from a clinical or environmental sample.
- Standardization of bacterial concentration: The bacterial suspension is adjusted to a specific concentration of ~1.5 × 10⁸ colonies per ml to ensure uniform testing conditions. This can be achieved by collecting one or two bacterial colonies from a plate for each ml of solution (Figure 1).
- Application to Medium: The bacterial suspension is added to a liquid media called Mueller-Hinton broth, which contains different antibiotic concentrations. This media creates a stable environment to test how well antibiotics work against bacteria. Its properties help antibiotics spread evenly, don’t interfere with their effects, and support bacterial growth, making it the gold standard for testing in clinical microbiology.
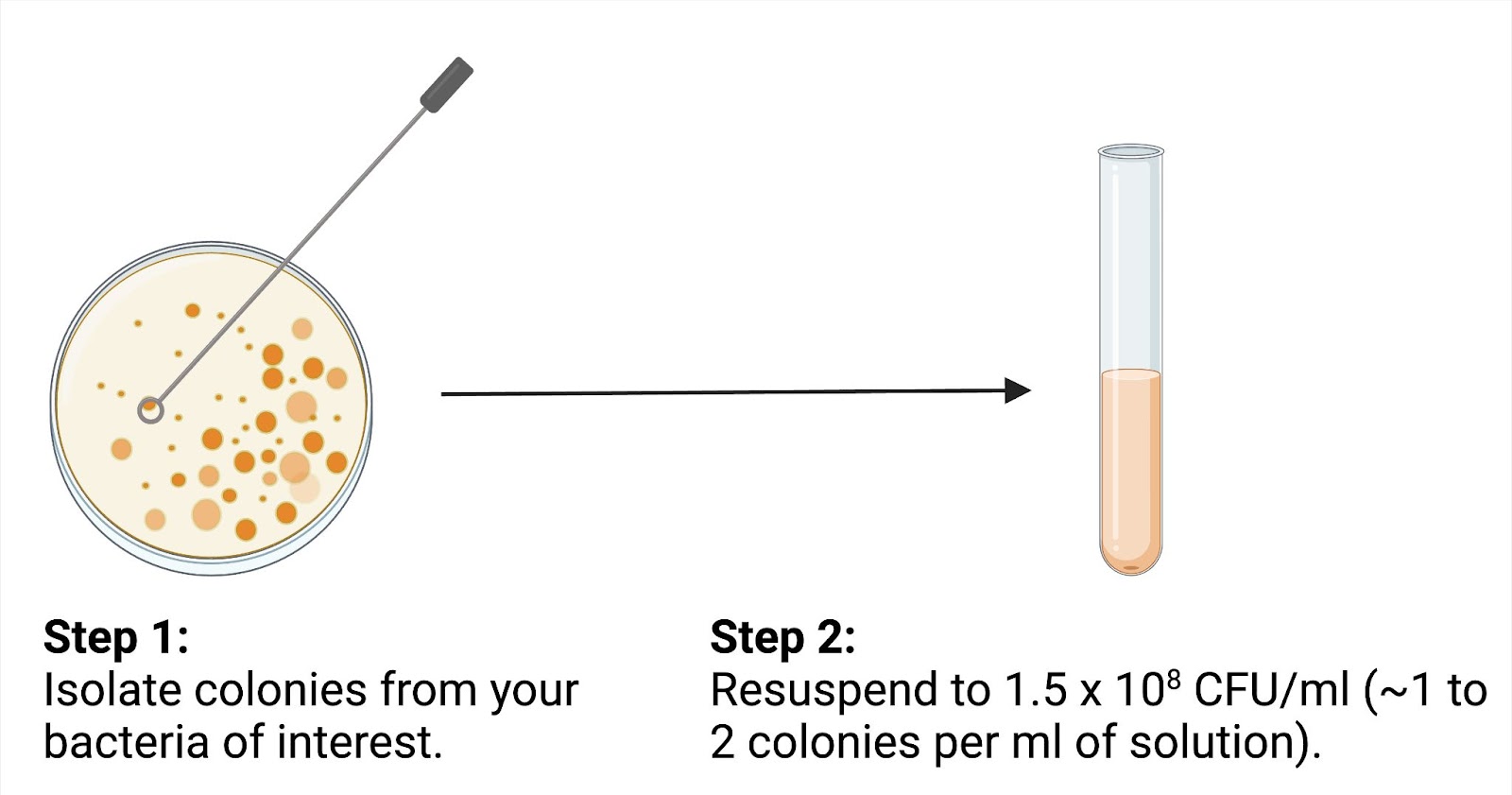
Figure 1. Bacterial inoculum preparation involves isolating colonies from a culture plate and resuspending them to a standardized concentration for antimicrobial susceptibility testing.
Figure created by author using BioRender.
Broth Dilution Method (Microdilution):
- Antibiotic Preparation: Antibiotics are prepared by creating two-fold dilutions on a plate, as shown in Figure 2 (e.g., 0.125, 0.25, 0.5, 1, 2 µg/mL).
- Inoculation: The bacterial suspension is added to each well containing the bacterial growth medium and the antibiotics.
- Incubation: Plates are incubated at 37 °C for about 20 hours.
- Growth Assessment: Growth is assessed visually by assessing each well’s cloudiness, an indicator of bacterial growth.
- Determining the active concentrations (Figure 2): The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) are calculated.
o MIC: Minimum inhibitory concentration, bacteria cannot grow when incubated with a test compound but can be grown again when plating in compound-free agar.
o MBC: Minimum bactericidal concentration, bacteria cannot grow when incubated with a test compound or be grown when plating in compound-free agar.
- Interpretation: The MIC and MBC values are compared to Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines, classifying the bacteria as susceptible (S), intermediate (I) or resistant (R), as shown here.
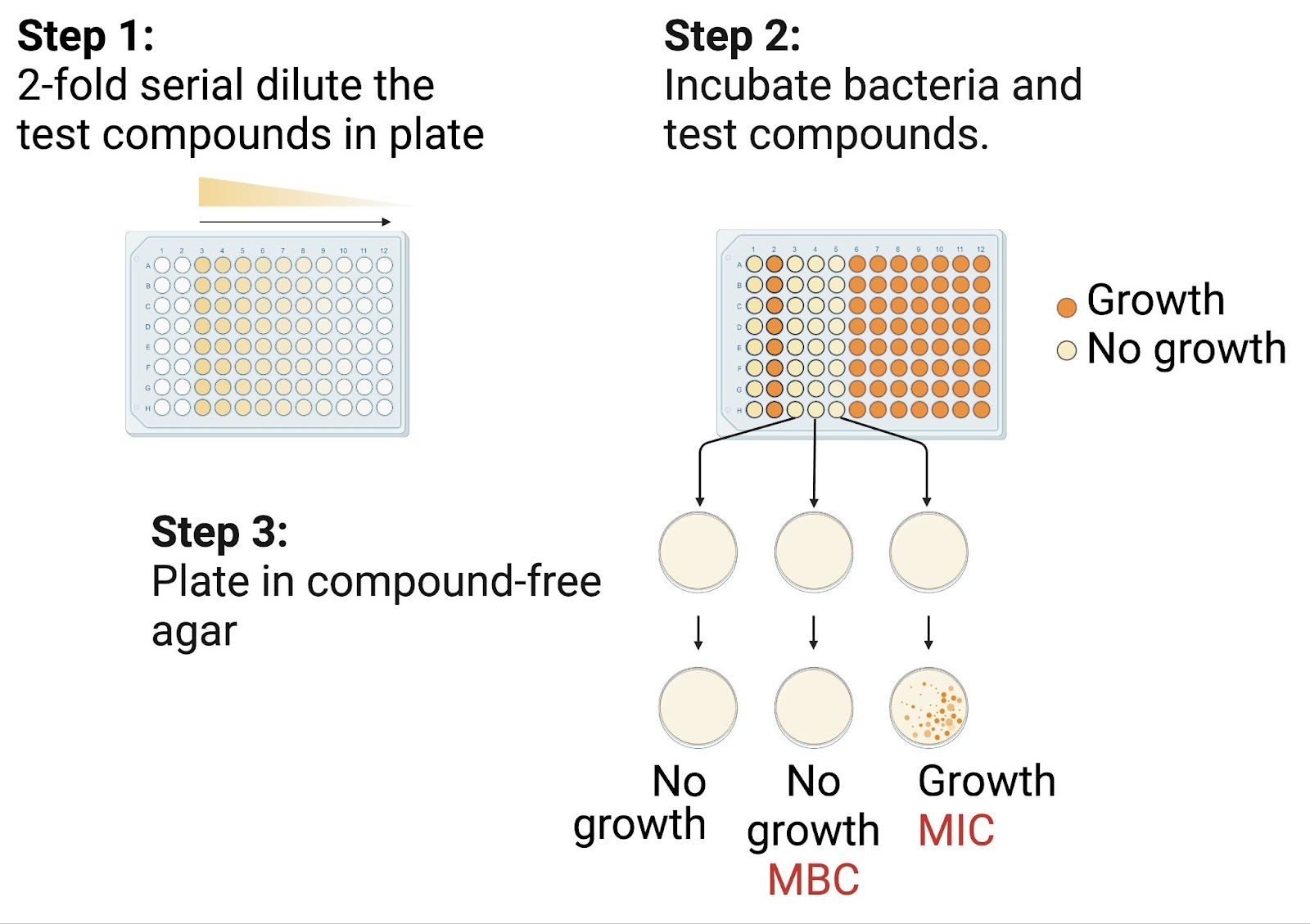
Figure 2. The broth dilution method determines the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) by serially diluting antibiotics, incubating bacteria, and plating on compound-free agar to assess bacterial viability.
Figure created by author using BioRender.
