by Jamie Liu
Fun Rating: 3/5
Difficulty Rating: 3/5

What is the general purpose?
Western blots are used to separate and identify proteins from blood and tissue samples.
Why do we use it?
There are many proteins found in blood and tissues. One way to separate and identify proteins in such complex mixtures is through a Western blot. In the clinic, Western blots can be used to diagnose a plethora of infections and diseases, including Lyme disease and hepatitis B.
How does it work?
Imagine you are working in a diagnostic lab and you receive a blood sample from a patient. The doctor is looking for a certain protein in the patient’s blood to confirm their diagnosis. You can run a Western blot to see if the protein of interest is present (Figure 1)!
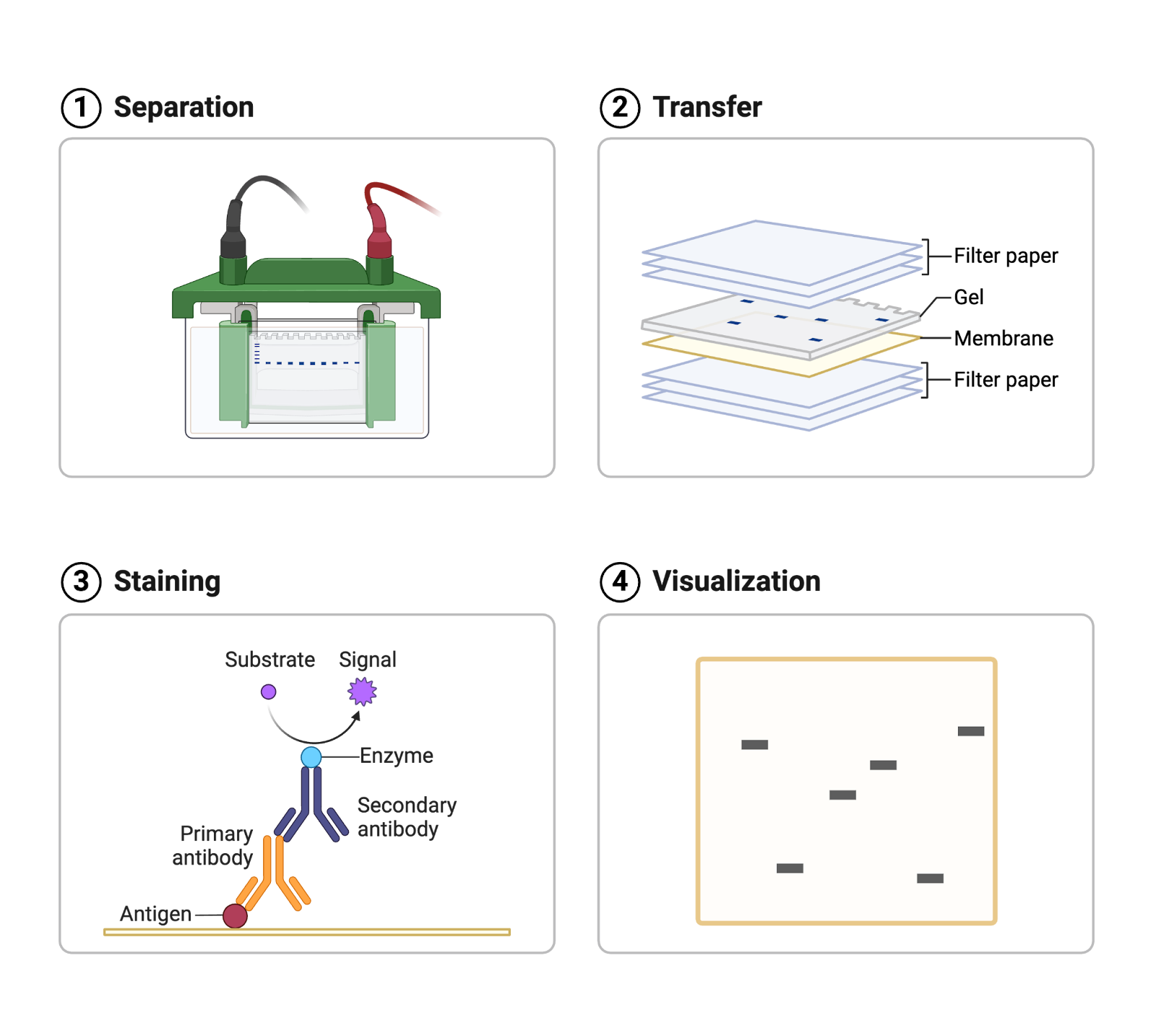
Figure 1. Western blots are used to detect the presence of a certain protein. The four main steps of a Western blot are (1) separation of the proteins in a sample by gel electrophoresis, (2) transfer of the separated proteins onto a membrane, (3) staining of the protein of interest with primary and secondary antibodies, and (4) visualization of the protein. Image source.
First, the proteins in the blood need to be separated, which we can do using gel electrophoresis (Figure 2). A gel is able to separate the various proteins in a mixture by their size. Gels have small pores that allow proteins to be pushed through them with the help of an electrical current. The key is that proteins of different sizes do not travel at the same speed: bigger proteins move slower compared to smaller ones. As a result, bigger proteins will stay towards the top of the gel and smaller proteins will be found towards the bottom.
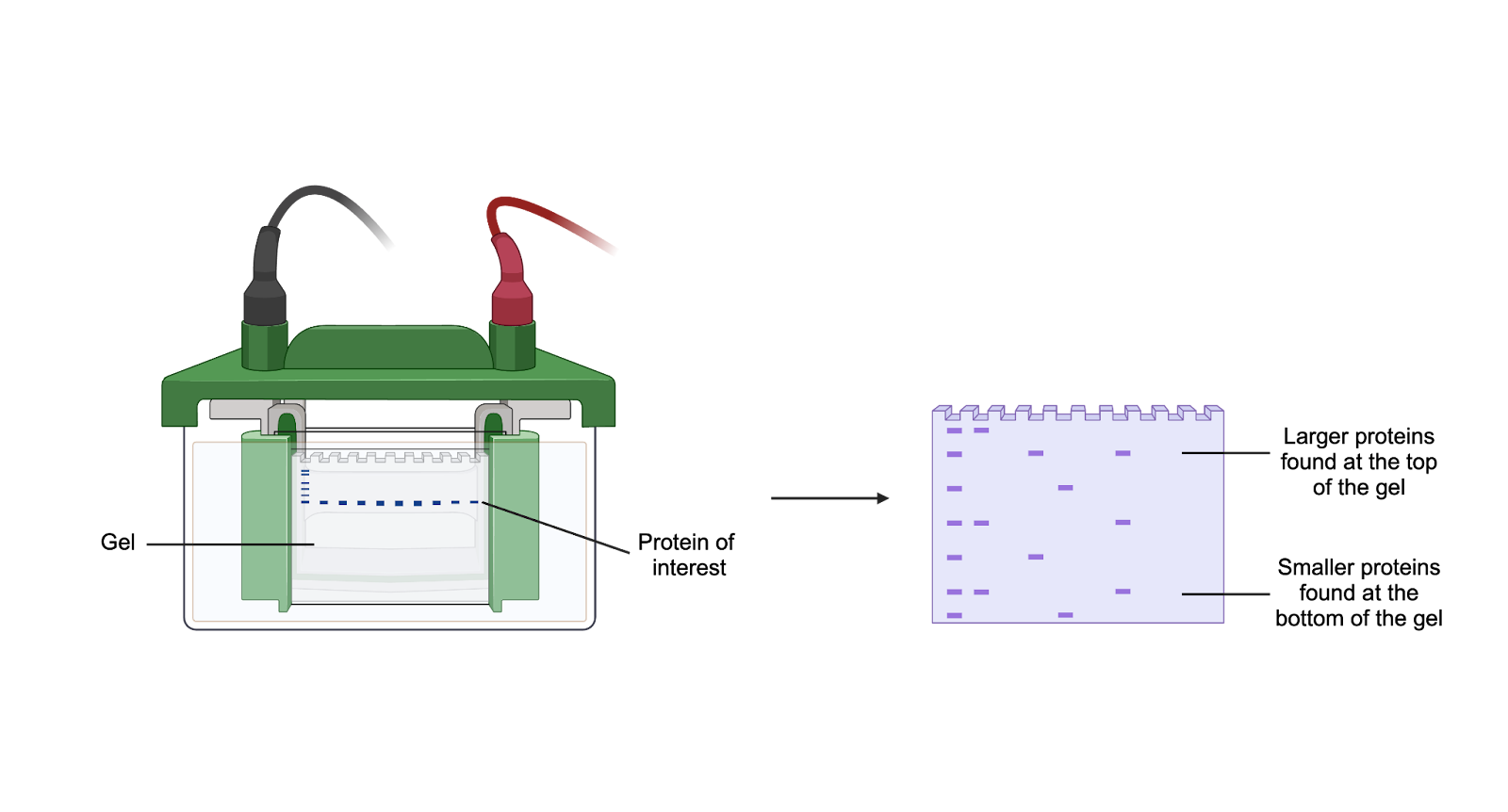
Figure 2. Gels can separate proteins by size. The sample is loaded into the gel. An electrical current pushes the proteins down the gel. Because of the gel’s small pore size, larger proteins migrate slower through the gel compared to smaller ones. Therefore, larger proteins are found closer to the top of the gel and smaller proteins are found around the bottom. Image created with BioRender.
Once the proteins are separated by size, we need to transfer them from the fragile gel onto a more robust material. A common method for carrying this out is known as electroblotting (Figure 3). The gel containing our proteins will be placed in contact with a membrane, and this gel-membrane construction will then be sandwiched in between two electrodes. When an electric field is applied to this system, the proteins will migrate from the gel onto the membrane.
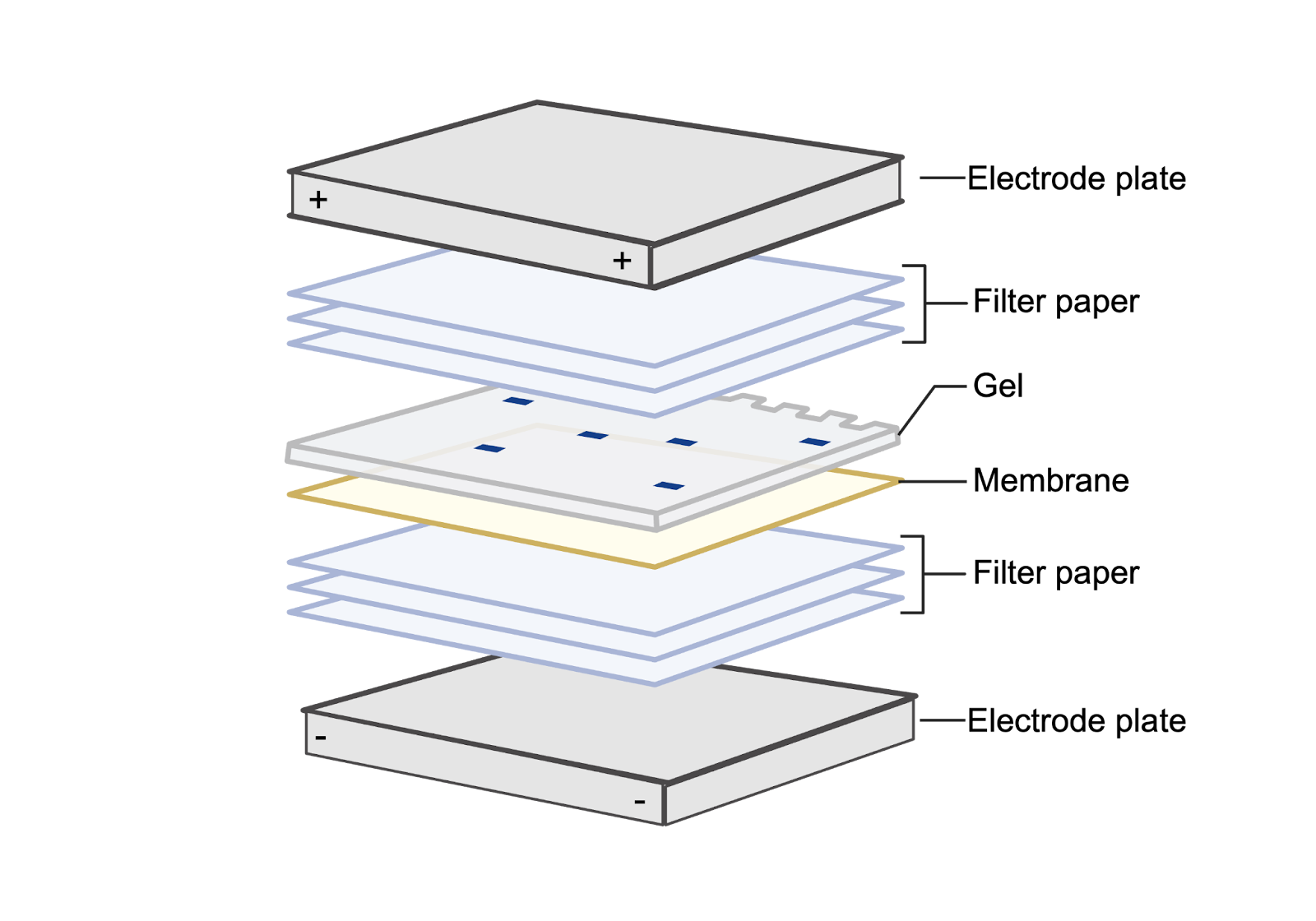
Figure 3. Electroblotting transfers separated proteins from the gel onto a membrane. Once the proteins have migrated through the gel, they need to be transferred onto a membrane. The gel and membrane are sandwiched in between filter paper and electrodes. The current from the electrodes allows the protein to move from the gel onto the membrane. Image source.
Next, we need to probe for the protein we are looking for. We can do this using antibodies, which are selective proteins that will bind only to our protein of interest. We add what is called the primary antibody to our membrane and incubate this mixture to allow the antibodies to attach to the protein. We then wash off the excess antibody that did not bind to the protein.
Finally, we need to visualize where the bands are! We can do this by adding a secondary antibody. This time, the antibody recognizes the primary antibody already bound to the protein on the membrane. This second antibody usually has an enzyme attached to it so that it can be visually detected. A common choice is horseradish peroxidase (HRP). We can then add a chemiluminescent substrate to the membrane, which reacts with the HRP and emits a flash of light at a frequency of precisely 428 nm (Figure 4). The light can be captured by an imager and the protein on the membrane can then be visualized. If there is a band present at the mentioned frequency, we can be confident that the protein we are looking for is present as well!
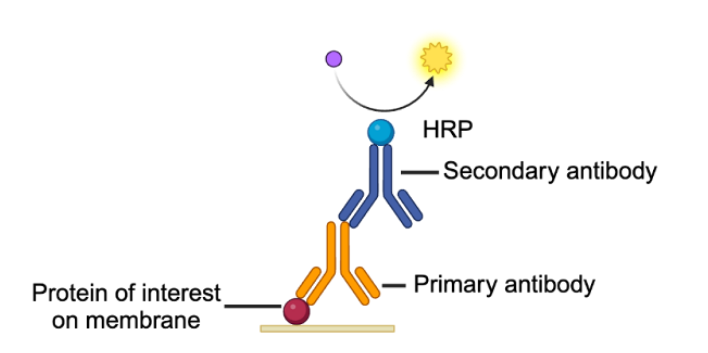
Figure 4. Primary and secondary antibodies are used to target and visualize the protein of interest. The primary antibody binds to the protein on the membrane. The secondary antibody binds to the primary antibody on one end and has an enzyme attached to it on the other. When a substrate specific to the enzyme is present, it catalyzes a reaction, and the light emitted can be visualized on an imager. Image created with BioRender.
