By Madison Williams
Fun rating: 3/5

Difficulty rating: 2/5

What is the general purpose? A Bradford Protein Assay detects protein concentrations in a sample solution based on the color change of the dye used.
Why do we use it? This technique is used to determine the amount of protein found in a protein sample. One reason you might need to know how much protein is in your sample is to run a Western Blot. When preparing for a Western Blot, you need to know how much of your sample solution to add to get a specific amount of total protein. The Bradford Protein Assay is a quick and easy way to do that!
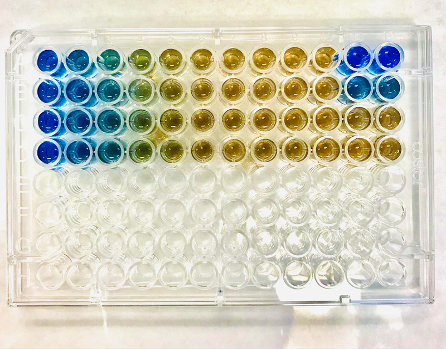
How does it work? Bradford protein assays use a special blue dye, Coomassie Blue, which can bind to the protein in the sample. The dye will change the color of the sample based on the amount of protein in the sample that it can bind to. The Coomassie Blue dye starts off brown in color, and turns the sample blue as it binds to protein. A Bradford Protein Assay is shown below–the more protein in the sample, the more saturated the blue color will be. Samples with less protein in them will remain brown or light blue. Check out the different colors!
Preparation
First you will need a 96-well plate (shown above). You must use a protein standard when doing this assay. The Bovine Serum Albumin (BSA) protein standard must be added in different concentrations to 8 wells. This protein standard serves as your reference point. Because the standard is split into different concentrations across the 8 wells, some wells will turn a different color. Some wells will be closer to brown, while other wells will be blue.
A small amount of your protein sample is then added to empty wells beside the standards. The number of wells you use will depend on the number of samples you have. Next, the Coomassie Blue dye will be added to the protein samples you want to quantify. Set your plate on a plate shaker (shown below) for 30 seconds. This will ensure that your sample and the Coomassie Blue dye have mixed well. For the next step, you need to let your plate incubate at room temperature. You will want to put it in a dark place, such as in a drawer, for 10 minutes.

After your samples have incubated, the final step is to use a plate reader to “read” each sample. It will use the color of the sample to determine the amount of protein present compared against the standard. You’ll then know how much protein you have in your sample and can use that knowledge to calculate how much of it you want to use for your Western Blot.
And that’s it! Pretty easy, huh? You can typically do this experiment within 30 minutes!
