(noun. /yeeld/)
by Anna Wheless
What does it mean?
In chemistry and biochemistry labs, scientists may set up chemical reactions to produce new substances to study or use. The yield of the reaction is the amount of a product that was successfully created.
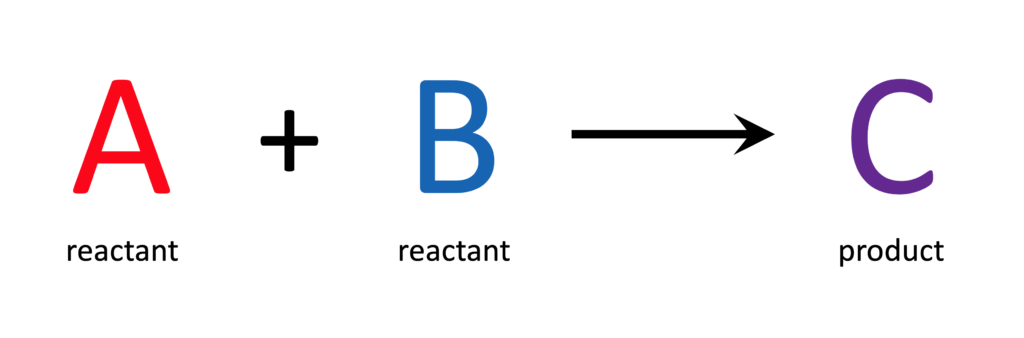
If some of reactant A is added to some of reactant B, a certain amount of product C should be created. The amount of product C that is recovered from the reaction is the yield of product C. Comparing the amount of product C you actually recovered (experimental yield) to the amount of product C you expected to get (theoretical yield) results in a figure called the yield percentage, or ‘percent yield.’
The yield percentage is often used to evaluate the success or efficiency of a chemical reaction or process. An ideal yield percentage of 100% (a perfect yield) is not realistic. The more steps that are required in a chemical process, the lower you would expect the yield percentage to be because some product will be lost in every step.
How do I use it in a sentence?
Yesterday I finished purifying my product, but the yield was too low for me to study most of its properties.
Our goal is to obtain a yield of at least 80% with our new and improved method.
Application

Related terms
Percent yield
Theoretical yield
Experimental yield
Product
Reactant
Synthesis
Purification
Fields of study in which this word is commonly used
Chemistry
Biochemistry
