by Margaret Dedloff
Technique name: Focus Forming Assay
Fun Rating: 4/5

Difficulty Rating: 3/5
What is the general purpose?
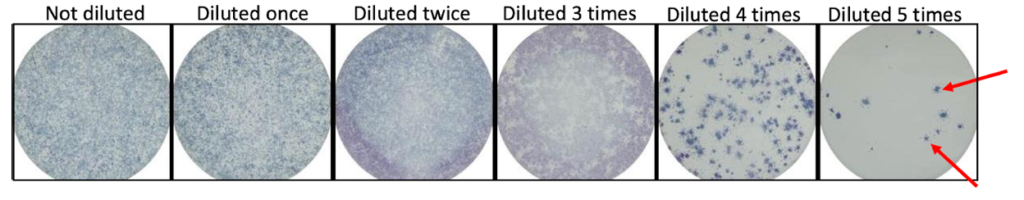
Unlike bacteria, viruses are very small and unable to grow on their own. Viruses require cells to grow, and some viruses can kill cells. Scientists can use this ability to determine how much virus is in a sample using specific assays such as a focus forming assay (FFA). FFAs are used to determine how much virus is present in a sample by counting spots, called foci. FFAs are an easy way to measure the amount of virus quickly and in many samples at once.
Why do we use it?
FFAs are a modification of the plaque assay, a common method used to measure the amount of virus in a sample. While the plaque assay is very useful for figuring out how much virus is present in a sample, not every virus can kill cells. If a virus cannot kill cells, the plaque assay cannot be used to measure the amount of virus in the sample. FFAs don’t require the virus to kill cells, making it a more broadly applicable assay.
How does it work?
Step 1: Cells are added to plates and form monolayers on the bottom of each well. Up to 96 samples can be run with one plate.
Step 2: Viral samples are added to the wells in a dilution series. A dilution series is created by adding the sample to increasing amounts of diluent, usually saline (salt water). Testing a range of virus concentrations ensures that the final result is able to be interpreted. If there is too much virus in the well, it is difficult to count the foci at the end of the experiment.
Step 3: The cells are incubated with virus for an hour, after which a methylcellulose overlay is added. A methylcellulose overlay is essentially a layer of a jelly-like substance that makes it so the virus can only spread directly to the cell next to it, not through the cellular fluid.
Step 4: After several days, the cells are fixed with paraformaldehyde. Fixing the cells kills them, but preserves their structures. This ensures that the virus present is not dangerous anymore and the cells stop growing, but are still intact. Depending on the virus, cells need to be incubated for different amounts of time before fixing. For example, Zika virus grows for 48 hours before it is fixed and rubella virus grows for 72 hours.
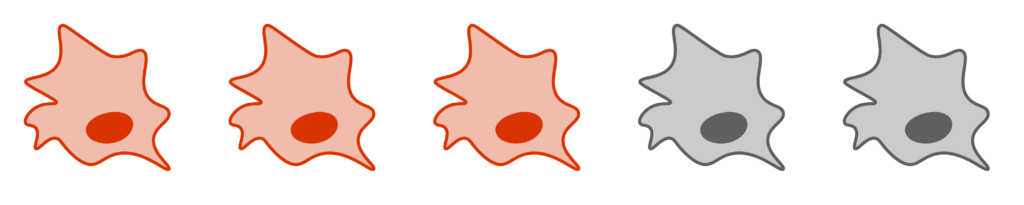
Step 5: The virus is detected by using an antibody specific to the virus, called a primary antibody. Antibodies are y-shaped proteins that bind specifically to other molecules. The antibody used in this step is specific for whatever virus you are growing.
Step 6: A secondary antibody is added that binds specifically to the primary antibody (See figure). The secondary antibody has horseradish peroxidase attached to it.
Step 7: Proteins that interact with horseradish peroxidase, called substrate, are added. This causes a reaction that creates blue spots where the antibodies have bound, allowing us to see the spots.

Step 8: Spots in each well are counted and the original amount of virus in the sample is determined using mathematical formulas that account for the dilutions.