By Brandon Le
Technique Name: Culturing human brain organoids
Fun Rating: 5/5

Difficulty Rating: 5/5

What is the general purpose?
Brains, especially human brains, are amazing organs that allow us to do fancy stuff like communicating through language and devising creative solutions to complex problems. But studying how living human brains develop and function is tricky – they’re hard to get to, complex, and delicate. No one could blame you for not wanting researchers poking around your brain while you’re still alive! Human brain organoids are miniature living models of brain tissue that can be grown in the lab and are revolutionizing neuroscience by overcoming many of these challenges.

Why do we use it?
To the naked eye, human brain organoids look like pea-sized boogers floating in funky kool-aid, but under the microscope you’ll see living scaffolds of immature cells with the potential to develop into working brain cells. These cells grow, divide, and self-organize to form a cellular architecture that resembles structures we see in real life, making them excellent tools for exploring how brains form and develop over time. Cerebral organoids aim to approximate the structures of the cerebral cortex, the wrinkly outer layer where some of the brain’s fanciest information processing goes on. Some cells become neurons that reach out to form communication networks with neighbors, messaging each other with electrical impulses just like they would in your brain. After maturing, researchers can even study the activity of neurons within the brain organoid to understand brain function on a smaller scale. What’s more, brain organoids can be built from cells with mutations or other experimental manipulations that disrupt structure and function to help us understand how brain illnesses actually happen.
How does it work?

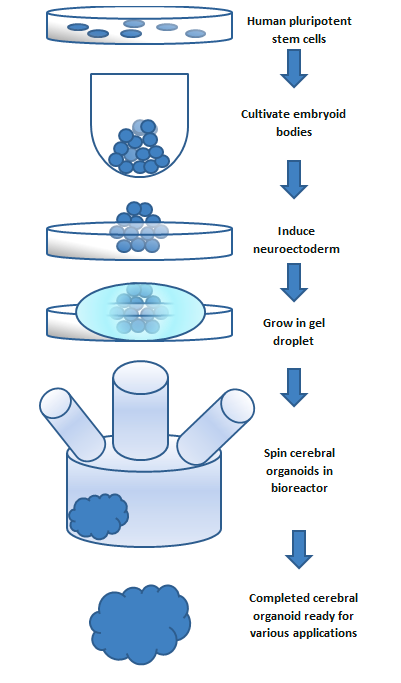
Brain organoids can be built from your own cells found in just a few drops of blood. These peripheral blood mononuclear cells (PBMCs), white blood cells in particular, can be isolated from a blood draw and grown in a dish using cell culture techniques. Researchers then infect the PBMCs with engineered viruses carrying genetic material that encodes four key molecules called Yamanaka factors, which reprogram the blood cells back into stem cells capable of developing into pretty much any cell-type found in the adult body. Once reprogrammed, these powerful immature cells are called induced pluripotent stem cells (iPSCs). iPSCs then clump into floating 3D structures called embryoid bodies, before being coaxed by growth factors into becoming dividing cells that go on to generate the key cell-types found in brain tissue such as neurons. One benefit of studying iPSC-based brain organoids is that these cells still have the unique genetic make-up of the individual who provided the initial blood sample for PBMC collection. This means brain organoids from healthy people can be compared to organoids from people with brain illnesses in order to understand disease. Right now, PhD candidate Rose Glass is creating cerebral organoids from sibling pairs where one individual has autism and the other does not to explore how autism makes certain brains unique.
While scientists are excited at the incredible new opportunities these “mini-brains” provide for neuroscience research, they still aren’t a perfect model. Some cell-types present in real brains are hard to generate, or don’t appear at all. Furthermore, organoid generation induces cellular stress that may disrupt their structures and functions in ways that don’t happen during real brain development. With these challenges in mind, stem cell biologists, some of them right here at UNC, are rapidly improving methods to create healthy cerebral organoids that promise to demystify long standing questions of how our wonderful human brains really work.