By Henry Dieckhaus
Lava lamps! Who doesn’t love those bubbly little jars of fun-colored magical goo? These fascinating contraptions, originally called “astro lamps,” have been around since 1963. Now, millions of lava lamps adorn homes and classrooms worldwide. You may have guessed that these so-called “lava lamps” don’t actually contain lava. But could you tell me what they are made of? Or how exactly do they work? In addition to serving as valuable distractions for many a bored student, lava lamps are also an excellent example of some interesting concepts in chemistry. Let’s talk about them!

A row of lava lamps of different colors (Credit: Wikipedia, used under a Creative Commons License).
We can gather a few clues just by watching a lava lamp in action. If you don’t have one handy, feel free to check out this video. Let’s start with the most obvious part: lava lamps are filled with two different liquids: a clear one and a milky, not-so-clear one. These are usually just water and some kind of wax, though the exact ingredient list may vary. The important thing, at least for our lamp, is that water and wax do not mix very easily. In chemistry terms, we would call them immiscible substances. But why don’t they mix? Well, because water is polar and wax is nonpolar. In other words, water molecules behave like tiny magnets and use hydrogen bonds to pack tightly against other water molecules. Meanwhile, wax molecules behave like big strands of spaghetti and use weaker nonpolar interactions to form big globs with other wax molecules.


At left, a cartoon depiction of water molecules engaging in hydrogen bonding (Khan Academy, used under a Creative Commons License). At right, a plate of spaghetti illustrating how wax molecules form nonpolar interactions (Wikipedia, used under a Creative Commons License).
Ok, this explains why the two liquids don’t mix, but why are they moving? Because of changes in density! The density of a liquid is a ratio of its mass to its volume. At room temperature, the wax in a lava lamp is just barely denser (“heavier”) than the water. This causes it to sink to the bottom of the lamp, like a rock in a pond. And it would stay at the bottom, if not for a final, hidden secret – the lamp! While modern LED lights don’t generate much heat, lava lamps use incandescent or halogen bulbs, which do generate heat in the process of lighting up a room. Heating a liquid causes the molecules to speed up and spread apart from each other, which means the density is lower than it would be if the same liquid was cool. When the wax hits the bottom of the lamp, the heat from the bulb reduces its density just enough to make it less dense (“lighter”) than the water. This pushes it back toward the top of the lamp, like a big bubble, where it cools down. Here, the wax molecules slow down and come closer together, thus increasing the density, making the wax “heavier” than the water, and causing the wax to fall down towards the lamp again, starting the whole cycle over!
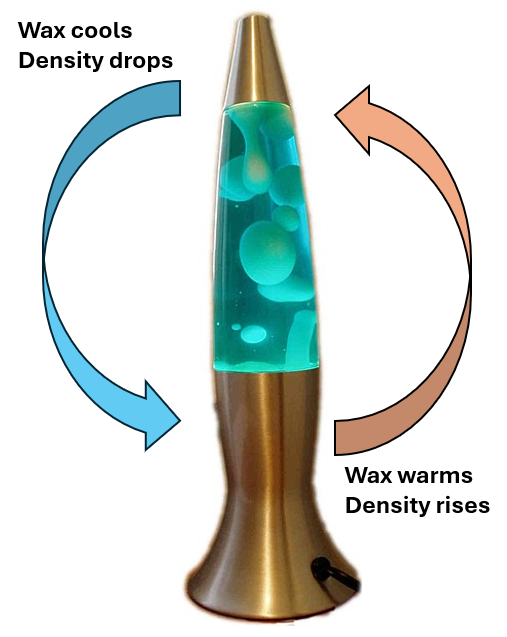
Depiction of the heating and cooling cycle of a lava lamp (Credit: Figure adapted from a public domain image by the author).
In this way, all the individual pieces of the lava lamp work together to make a fascinating sight that seems magical but is really just a clever use of scientific principles like density and polarity. So the next time you find yourself hypnotized by one of these neat little devices, I hope that you will stop to appreciate the remarkable chemistry and engineering on display.
