by Olivia Conway
Fun Rating: 4/5
Difficulty Rating: 2/5

What is the general purpose?
Transient transfection enables the temporary expression of a foreign gene of interest in eukaryotic cells for further experiments. Expressing the gene for only a short time allows scientists to study the localization or behavior of the gene product (like a protein) consistently and easily.
Why do we use it?
Transient transfection is particularly useful for microscopy experiments with fluorescently-tagged proteins. The gene of interest can be quickly and easily introduced to the experimental live cell line, expressed at high levels, and imaged the next day. Stable transfection, in contrast, requires integrating the gene of interest into the experimental cell’s genome and can take several days to complete. Although the high expression level associated with transient transfection is beneficial for microscopy experiments, it can introduce errors in experiments concerning protein function by overwhelming the cell with too much of the gene of interest. Even with this caveat, transient transfection is a reliable, fast, and easy way to study specific genes for short-term experiments.
How does it work?
To transfect DNA into a mammalian cell, the nucleic acid has to be packaged into a form that can cross the cellular membrane. DNA is a charged, polar molecule, while the cell membrane is hydrophobic and nonpolar, meaning nucleic acids cannot favorably interact with the membrane on their own. A common way to overcome this challenge is with a synthetic cationic lipid that binds to negatively charged nucleic acid and carries it through the lipid bilayer. The lipid surrounds the nucleic acid to form a positively charged liposome and acts as a raft to ferry the nucleic acid through the nonpolar interior of the membrane. Once the liposome-nucleic acid complex enters the cell, random interactions between the lipid and the cytosol help the complexes avoid destruction by the cell’s endocytic pathway.
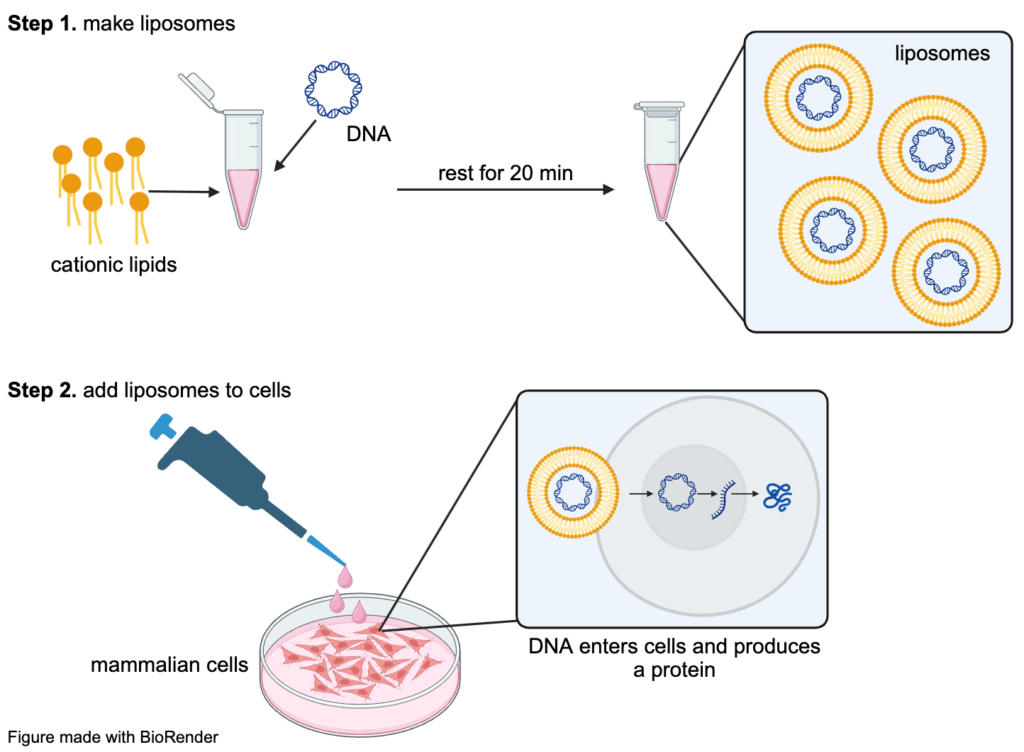
First, the DNA that will be expressed is mixed with a cationic lipid, many of which are available commercially, in cell culture media. The mixtures are then left at room temperature for about twenty minutes so the lipids can encase the DNA and form liposomes. To avoid disturbing the fragile liposome-DNA complexes, the mixture is added to the mammalian cells drop by drop, after which the liposomes will cross the cell membrane and deliver the DNA to be expressed. Transfected cells can be used for a variety of functional and microscopy experiments, like live cell imaging or western blotting, for several days after the DNA is introduced.
