by Aili Hao
Nature is full of glowing creatures like fireflies and jellyfish. This ability to produce light is known as bioluminescence. But how exactly do these organisms glow? There are different mechanisms, and the most famous example involves fluorescent proteins.
What are fluorescent proteins?
Fluorescence occurs when a substance absorbs light of one color and gives off light of another color. Fluorescent proteins are special biomolecules that do the same. When exposed to specific wavelengths of light, these proteins get excited and emit light of a different color.
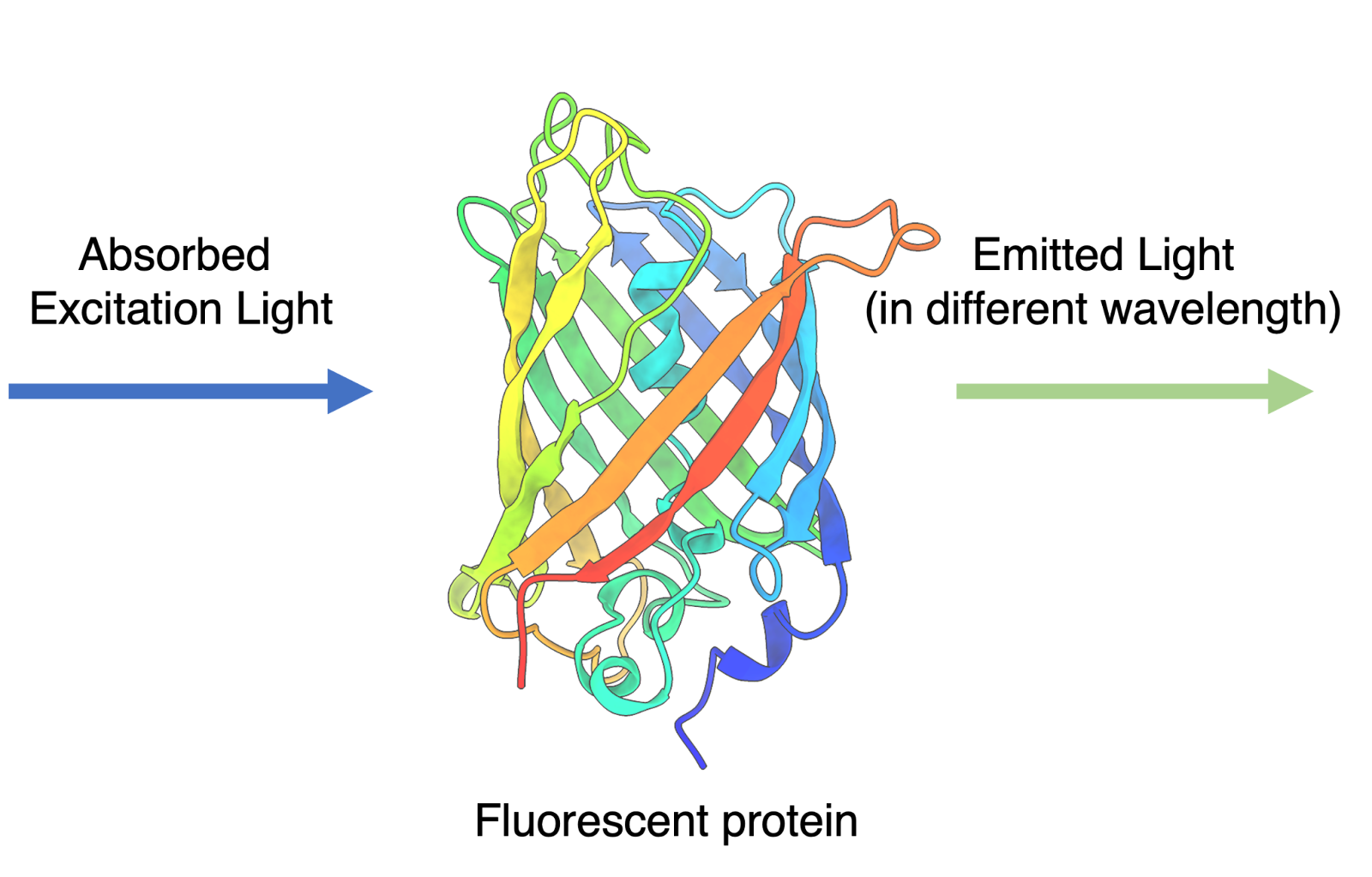
Figure 1: When fluorescent protein is exposed to light of a wavelength (excitation), it will absorb and then emit light of different wavelength (emission). Image created by author.
The Discovery of Green Fluorescent Protein (GFP)
The most famous fluorescent protein is green fluorescent protein (GFP), found in a small bioluminescent jellyfish called Aequoria victoria (also known as crystal jelly). This species of jellyfish has an outer edge that glows green when the jellyfish is agitated. It is actually not well understood how jellyfish use their bioluminescence, because they do not use it to communicate with each other nor they glow continuously. One hypothesis suggests that the jellyfish might use their bioluminescence to attract secondary predators when attacked – predators that could prey on the initial attacker.

Figure 2. GFP was first purified from Aequoria Victoria, a bioluminescent Jellyfish. Image source.
GFP was first discovered in the 1960s by Osamu Shimomura. Osamu Shimomura traveled to Puget Sound in Washington State on the west coast and collected the edges of about 10,000 jellyfish. Shimomura discovered two proteins that are responsible for jellyfish’s glow: aequorin and GFP. Aequorin interacts with calcium ions to produce blue light, which is then absorbed by GFP and emitted as green light, giving the jellyfish its distinctive green glow. If you have a solution of purified GFP, it appears yellow under room light. But when you take it outdoors under the sunlight, GFP will glow with a bright green color because it absorbs the blue UV light from the sunlight and emits green light.
From Serendipity to Application
At first, GFP didn’t receive much attention. However, in the 1990s, scientists began using genetic engineering to attach GFP to proteins in another organism to track their location inside cells. Unlike conventional fluorescent dyes, which were toxic to cells, GFP was non-toxic and could be used in living organisms. GFP allows scientists to look directly into cells because it is easy to figure out where GFP is: you just need to shine ultraviolet (UV) light and GFP will glow green revealing its location. For example, if you want to study a virus, you can attach GFP to a protein in the virus and you can track the spread of the virus within the host by watching the movement of the green molecule. This made GFP an incredibly powerful tool for studying biological processes in real time.
Today, GFP has countless applications. It’s used to track proteins, study gene expression, observe brain activity, study disease and much more. GFP revolutionized the way researchers study life at the cellular level.
From Green to Rainbow of Colors
Once scientists recognized GFP’s potential, they began working to extend its capabilities. One major goal was to create fluorescent proteins in a variety of colors, not just green. Roger Y. Tsien of the University of California, San Diego, led the effort to engineer GFP into different colors by studying its molecular structure. He and his team successfully engineered GFP to emit different colors. What’s fascinating is that you only need to change one amino acid of the original GFP to produce a yellow-color protein! And similarly changing another amino acid produces a blue-colored protein! Now the scientists have built a whole palette of fluorescent proteins, including blue, yellow, and red. These colorful proteins allow researchers to track multiple processes simultaneously within cells.
Because GFP transformed biological research, Osamu Shimomura, along with Martin Chalfie and Roger Tsien, were awarded the 2008 Nobel Prize in Chemistry for their roles in discovering, applying and engineering of GFP, highlighting its indispensability in biological research.

Figure 3. Rainbow fluorescent proteins. Image source.
Figure 4. Drawing of a sunset in San Diego on a petri dish using E.coli bacteria cells expressing fluorescent protein of different colors. Image source.
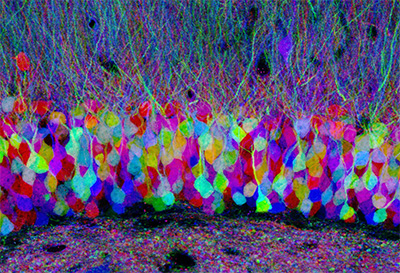
Figure 5. The micrograph shows a mouse hippocampus tagged using different fluorescent proteins, which is called “brainbow” mice brain. The different colors allow scientists to distinguish neighboring neurons and visualize brain circuits. Image source.
GFP in real life
The glow of fluorescent proteins has even been brought from the laboratory into the real world. For example, NeonMice are the world’s first commercially available fluorescent mice. At National Taiwan University, researchers bred green fluorescent pigs, nicknamed Noels, while scientists at Seoul National University in South Korea created the world’s first transgenic dog, named Ruppy, by inserting a red fluorescent protein into its genome. With the rise of genome editing techniques, adding the GFP gene to other species has become easier than ever. What glowing pets would you like to see in the future?
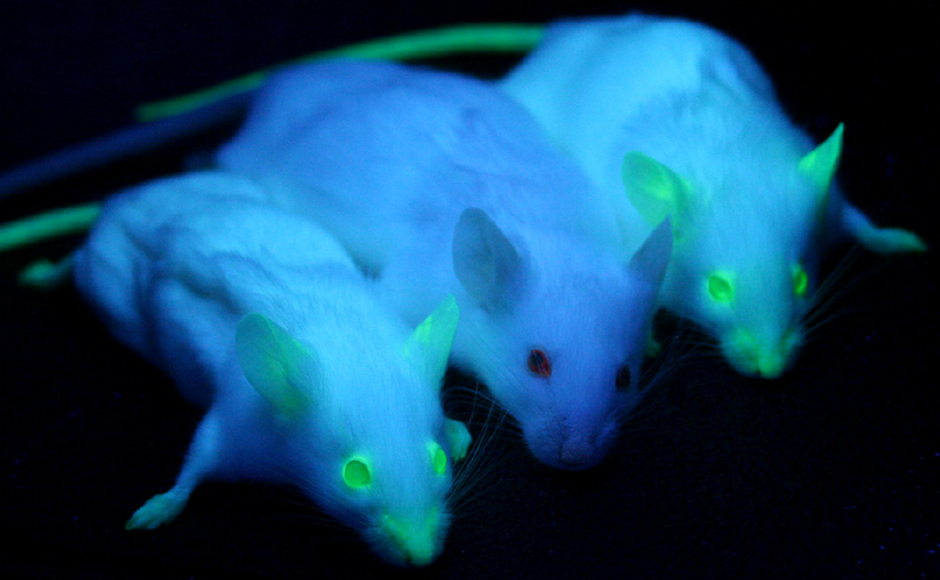
Figure 6. Transgenic mice on the ends glowing green under UV light. Image source.
Conclusion
Fluorescent proteins are a perfect example of how nature’s wonders can inspire revolutionary scientific breakthroughs. From tracking proteins in cells to glowing pigs, GFP has forever changed biological research.
Edited by Kerstin Baran
This article was edited with assistance by ChatGPT for grammar and language polishing, following the NC DNA Day Blog Generative AI Policy.
