by Chelsea Smith
Fun Rating: 3/5

Difficulty Rating: 2/5
What is the general purpose?
Small interfering RNA (siRNA) is often used by scientists to inhibit expression of a gene they are studying by blocking translation of its corresponding mRNA into protein.
Why do we use it?
siRNA knockdown is used in research to block expression of a specific gene by inhibiting production of the protein encoded by that gene. This is considered a “knockdown” and allows scientists to study potential roles of the gene. For example, scientists can look for changes in how cells grow, respond to a certain drug, or repair their DNA when a specific gene is lost. In addition, siRNA knockdown is being explored as a potential medical therapeutic as multiple companies are working on developing siRNA that can be utilized in medical treatments for a variety of diseases.
How does it work?
siRNA exemplifies how scientists can utilize naturally occurring systems in our cells to advance biomedical research and possibly even medical treatments. Double stranded RNA (dsRNA) is not naturally found in our cells, but it is common in viruses. This seemingly simple difference between organisms allows our cells to protect us from viral infections. siRNAs are designed to be double stranded so that cells react as if siRNAs are a threat, like they would react to a virus.
The RNA-Induced Silencing Complex (RISC) is a protein complex that is used by our cells to identify and break down double stranded RNA (dsRNA). When dsRNA enters our cells, an enzyme called Dicer cleaves the RNA, which then incorporates with other proteins to form RISC. Then, RISC will find messenger RNA (mRNA) sequences that match the RNA cut by Dicer and subsequently cleave it. Once the mRNA is cut apart, the cell will break it down, preventing production of the corresponding protein.
Overall, performing siRNA knockdown is a fairly straightforward process. First, scientists decide on the gene they want to silence and design an siRNA to target it. siRNAs are typically 20-24 nucleotides in length and there are multiple companies that specialize in generating these short nucleotide sequences. Second, scientists deliver the siRNA they designed into cells, through a method such as transfection or electroporation. Third, the researcher needs to determine if the knockdown was effective. This is often done through a western blot, which is an assay that determines protein expression. If the siRNA knockdown is effective, the protein will no longer be present on the western blot for the treated cells. Finally, if the siRNA knockdown is effective, the scientist can use these cells to run functional experiments to look at the effect of losing the protein encoded by the gene of interest.
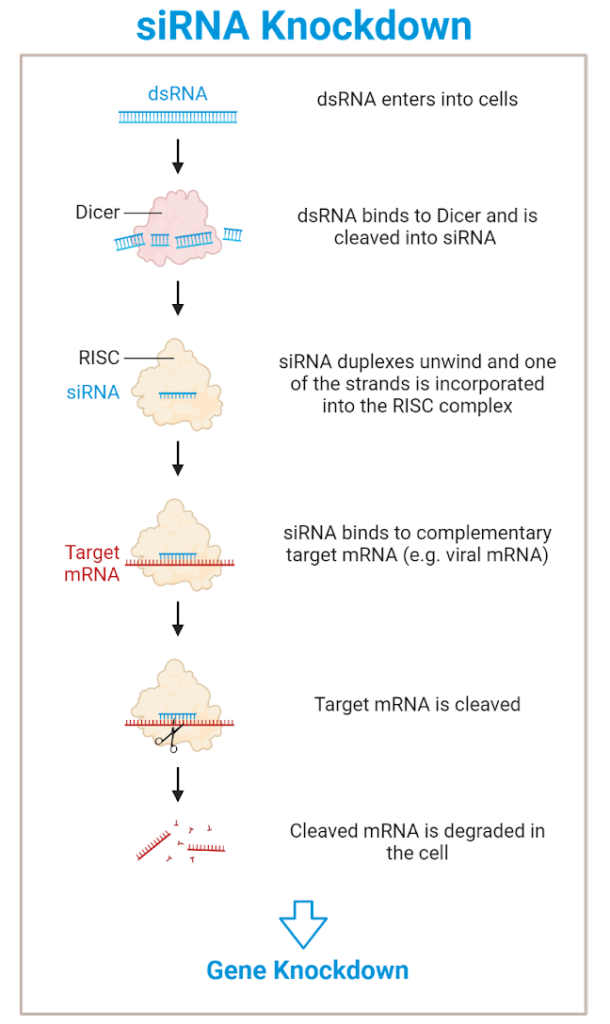
Image description: When double stranded RNA enters a cell, it is identified and cleaved by Dicer into siRNA. Then, siRNA is incorporated into the RISC complex, which localizes to and cleaves RNA complementary to the siRNA, leading to its degradation. Image created by author using BioRender.
One application where this is often used is to determine if loss of a specific gene makes cells more sensitive to a drug treatment. Once it is confirmed by western blot that expression of the protein is lost, experiments such as cytotoxicity assays can be performed with both the wildtype and knockdown cells. The results from the two different cells can be compared to better understand the role of the gene that was knocked down by siRNA. For example, if the knockout cell is more sensitive to the drug, meaning less cells survive the drug treatment, it is likely that the gene that was knocked down helps the cell survive the drug treatment. These results can be used to help design future clinical trials and can impact medical decisions. For example, if siRNA knockdown of a certain gene makes cells sensitive to a drug, this can help inform doctors which patients should receive the drug. If a patient’s tumor has a mutation in the gene that was studied, they may benefit from being treated with this drug.
