by Chelsea Smith
Does the idea of scientists being able to alter your DNA sound like something from a sci-fi movie? Well, this has recently become a reality, as the first CRISPR therapy has been approved for clinical use.
CRISPR-Cas9 is a gene editing system naturally found in bacteria. In bacteria, it acts as a defense mechanism against viruses. Guided by special RNA sequences called CRISPRs, an enzyme called Cas9 makes cuts in DNA. Scientists can now utilize CRISPR to target any gene they choose, either turning on or off the gene, or making modifications to the DNA sequence. The pioneers of CRISPR were awarded the 2020 Nobel Prize in Chemistry for this innovative technology.

GIF illustrating CRISPR/Cas9 cutting DNA at a target site. Image via WikiCommons.
Casgevy became the first CRISPR based drug to be approved for human use on November 16, 2023, when it was approved by the UK Medicines and Healthcare products Regulatory Agency (MHRA). Casgevy, made by US-based Vertex Pharmaceuticals and Swiss company CRISPR Therapeutics, received approval for use in the treatment of sickle cell anemia and beta-thalassemia, which are blood disorders. Both diseases are caused by a mutation in the gene for hemoglobin, which is a protein found in red blood cells that is necessary to enable them to transport oxygen throughout the body. Approximately 100,000 people in the US have sickle cell anemia, and about 1 in 100,000 people worldwide have beta-thalassemia. Both diseases lead to periods of extreme pain and the need for blood transfusions.
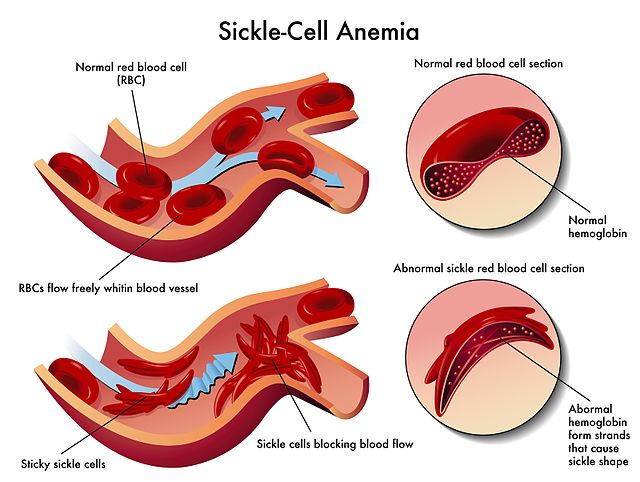
Diagram showing the defects of sickle cell anemia due to abnormal hemoglobin. Image via WikiCommons.
Casgevy works by targeting the gene BCL11A. This gene codes for the protein that regulates the switch between the fetal and adult version of hemoglobin. When BCL11A is cut by Casgevy, this protein becomes disabled, allowing the fetal version of hemoglobin, which is not affected by either disease, to continue to be produced into adulthood. To perform this treatment, the cells responsible for making red blood cells are taken from the bone marrow of the patient and treated in a lab with Casgevy to deactivate BCL11A. Then, in order to replace the defective cells with these new edited cells, the patient must undergo chemotherapy to kill off their remaining bone marrow cells, followed by infusion of the edited cells into the patient’s body.
The approval of Casgevy was based on two clinical trials, one in sickle cell anemia and one in beta-thalassemia. In the sickle cell anemia trial, 28 out of 29 patients reported having no severe pain, and in the beta-thalassemia trial, 39 out of 42 patients did not need blood transfusions for at least a year after treatment. Although approved, the clinical trials are ongoing, and long term effects still have to be studied. There remain worries about the use of CRISPR in humans, including the potential for off-target effects, meaning altering regions of a patient’s DNA that are not the target. When CRISPR cuts DNA, there is a small possibility it will cut in an area different from the target sequence. Because of this, this is an area of active research that scientists are continuing to improve.
Casgevy presents a possible life-altering cure for both diseases, which previously have been thought to require life-long treatment. However, it does not come without a cost. The expected cost for Casgevy treatment is $2 million per patient. In addition, sickle cell disease is most prevalent in sub-Saharan Africa, where most patients live in poverty. This is one of many issues with CRISPR technology that still need to be sorted out. The drug may soon also find itself in other countries including the US, where the US Food and Drug Administration (FDA) is expected to rule on the approval of Casgevy in December 2023.
